Technology Insights
Data Integrity
Evidence
Analysis
Transcription
Observations
Data Entry & Check
Conclusions
Numerical Analysis
Recommendations
#1 Evidence
Raw Data
E-Data
Log Book
Wet Ink Sheets
#2 Analysis
Data Entry
Numerical Analysis
Run graph check
X,Y,Z Graphs
Trend Lines
Data integrity is a worthy subject, if dull to most. Evidence must be stored as raw data, ready for re-inspection. UPC use wet ink as the preferred method, and raw e-data in a vault. The raw data becomes couplets, triplets or more; then graphed for trend analysis. To present data that is accurate and correct ‘beyond reasonable doubt’ is a hard task, that only a few do with full understanding and grace.

Door frame height readings recorded in a ‘log book’ in indelible ink over ten years. A rule was used to transpose the marks into heights for the two growing children, date
The measurement error in heights is ±1mm at best, depending on whether the sharpie mark was placed with the flat surface at 90° to the frame. Transcription error ±0.5mm.
Checking: A date transcription mistake was found when graphing versus time on P trace.
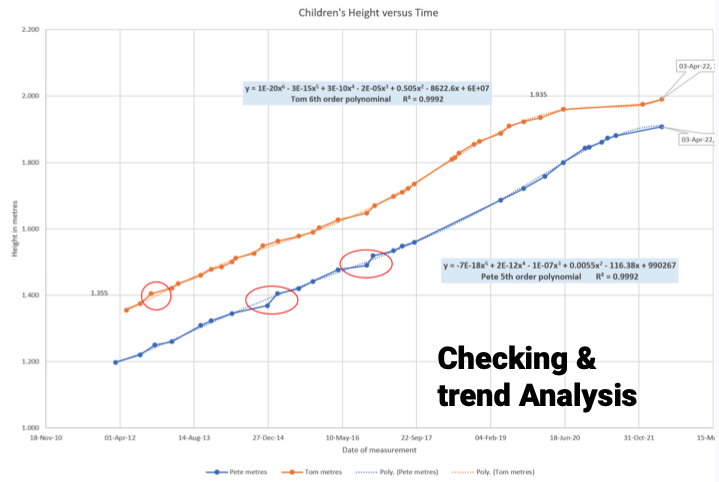
Q: Compare the two children’s height with time? Will P be taller than T when the same age?
O: T will not get much taller as his growth is slowing. P is still growing taller over the last 12 months.
O: The data predicts that P will not catch up T over 502 days.
C: Past performance does not predict future performance… R: Keep taking data…
