
Patent infringement Diabetes CSII Patch Pump
UPC provided expert witness on a case tried in the UK High Court – Patent Division. The subject matter was diabetes type 1 (T1) treatment using Continuous Subcutaneous Insulin Infusion (CSII) rather than Multiple Daily Injections (MDI). {Type 1 diabetics rely on daily insulin delivery to avoid blood sugar lows or sugar highs, both of which have damaging consequences.}
Insulin delivery is literally a ‘life-saver’ for those in need, and consequently the University Of Toronto patented their methods, but gave free licences to companies who could mass-produce the product to the UOT requirements.
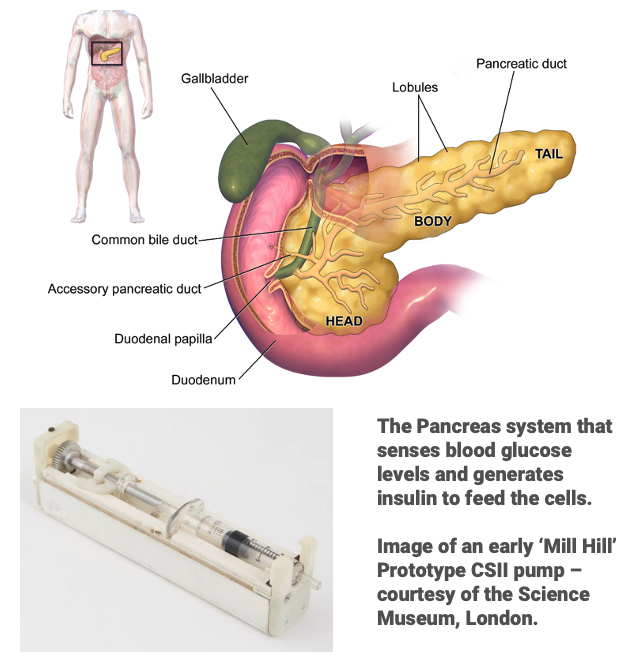
In the 1970’s the ‘Mill Hill’ prototype CSII pumps were being tested at Guy’s Hospital, London. These small portable devices contained a small syringe and a battery powered driver with a fixed basal rate for insulin delivery, but they also had a fixed 8 fold higher delivery for prandial use.
The pump technology matured into belt-wearable marketed devices in the early 1980’s with Medtronic Minimed (USA) and Diesetronic (GER) and others later offering competing devices with different strengths, and weaknesses. Typically the CSII pumps were worn belt mounted, with the infusion site in the stomach area.


Patent infringement Diabetes CSII Patch Pump
UPC provided expert witness on a case tried in the UK High Court – Patent Division. The subject matter was diabetes type 1 (T1) treatment using Continuous Subcutaneous Insulin Infusion (CSII) rather than Multiple Daily Injections (MDI). {Type 1 diabetics rely on daily insulin delivery to avoid blood sugar lows or sugar highs, both of which have damaging consequences.}
Insulin delivery is literally a ‘life-saver’ for those in need, and consequently the University Of Toronto patented their methods, but gave free licences to companies who could mass-produce the product to the UOT requirements.
In the 1970’s the ‘Mill Hill’ prototype CSII pumps were being tested at Guy’s Hospital, London. These small portable devices contained a small syringe and a battery powered driver with a fixed basal rate for insulin delivery, but they also had a fixed 8 fold higher delivery for prandial use.
The pump technology matured into belt-wearable marketed devices in the early 1980’s with Medtronic Minimed (USA) and Diesetronic (GER) and others later offering competing devices with different strengths, and weaknesses. Typically the CSII pumps were worn belt mounted, with the infusion site in the stomach area.
UPC provided a report, including Common General Knowledge, contrasting the two competing CSII systems.
In the early 2000’s insulin pumps took a step forward to allow T1’s to stick the pump directly to the skin – these types were known as patch pumps, since they more resembled a patch of sticking plaster than the belt-mounted systems.
This legal case saw an early patch pump manufacturer challenging a later, smaller patch pump manufacturer that it alleged infringed a patent it owned. The more recent product can be seen in the right-hand photograph. This pump is small and light enough to be worn on the upper arm, as well as in the more traditional stomach site. It has two operational buttons on it, as well as a multi-functional controller seen in the background.
To the left of the pump can be seen a plastic syringe with a Luer connector aspiration needle added that has been bent through 90 degrees. This prop was not used in Court for legal reasons, but shows the internal principal construction of a patch pump liquid path to the subcutaneous layer.


Cross-examination is a challenge, like no other. Aggressive questioning on many hundreds of pages of documents - a university viva by an angry professor…
This UPC archive photograph shows a typical bundle of twelve document folders used in a legal case of this type. The papers on top show the level of documentation produced for the first and second reports in legal cases of this type by UPC.
The super-imposed high-definition photograph shows a co-moulded elastomeric seal fitted to the disposable ‘syringe’ part of the Solo patch pump. This seal provides water ingress sealing for the multi-use miniature syringe driver system, which also contains the electronics. The syringe and driver are combined together by the user to provide insulin supply for upto a few days use for those requiring lower insulin delivery.
The third generation patch pump is clipped onto a cannula plate that provides the final physical element to allow insulin to be delivered sub-cutaneously.
The High Court found that a. the Solo device did not infringe the patent, and b. that the patent the Solo device was claimed to infringe, was invalid.

