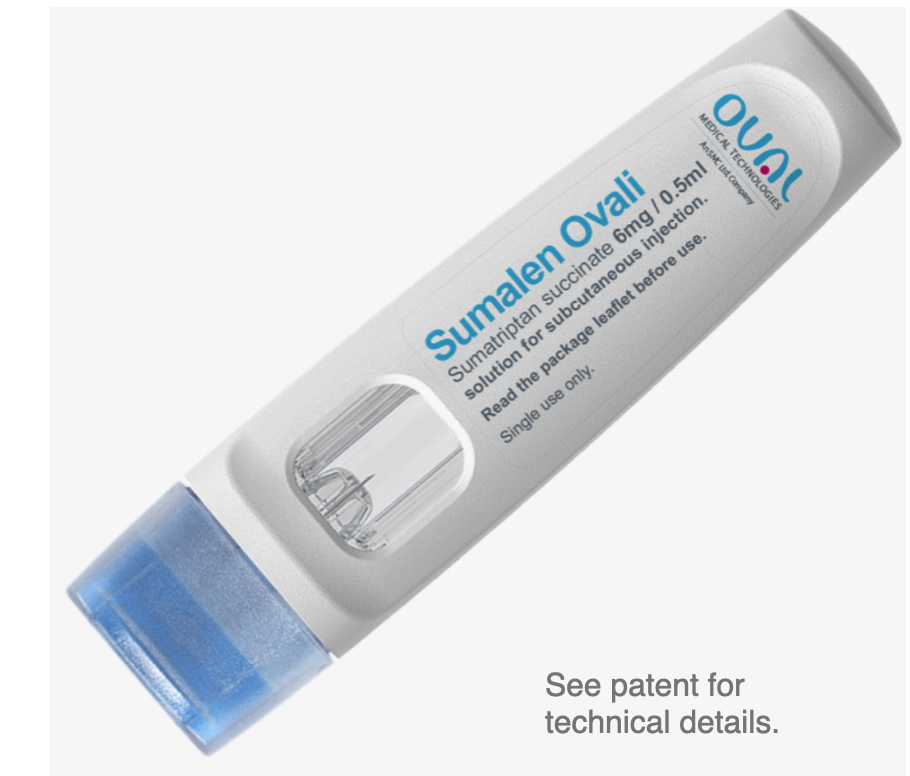
Autoinjector for rapid migraine treatment.
UPC worked as the Interim Head of Development and Engineering Director for six months during 2015.
The key responsibility was to ensure the Sumalen migraine treatment project was ready for regulatory approval, with complete technical files for all the critical to quality (CTQ) functions.
Some items simply required a report writing, but other fine details were not fully understood by the design team who had created the injector in the first instance. So first-principles engineering was required supported by collection of test data to verify the hypotheses.
By the end of the project Sumalen was technically ready to go as a design – fully frozen for manufacture as UPC like to call it.


Autoinjector for rapid migraine treatment.
UPC worked as the Interim Head of Development and Engineering Director for six months during 2015.
The key responsibility was to ensure the Sumalen migraine treatment project was ready for regulatory approval, with complete technical files for all the critical to quality (CTQ) functions.
Some items simply required a report writing, but other fine details were not fully understood by the design team who had created the injector in the first instance. So first-principles engineering was required supported by collection of test data to verify the hypotheses.
By the end of the project Sumalen was technically ready to go as a design – fully frozen for manufacture as UPC like to call it.
Beautiful design, Great human factors requiring detail engineering to get to market
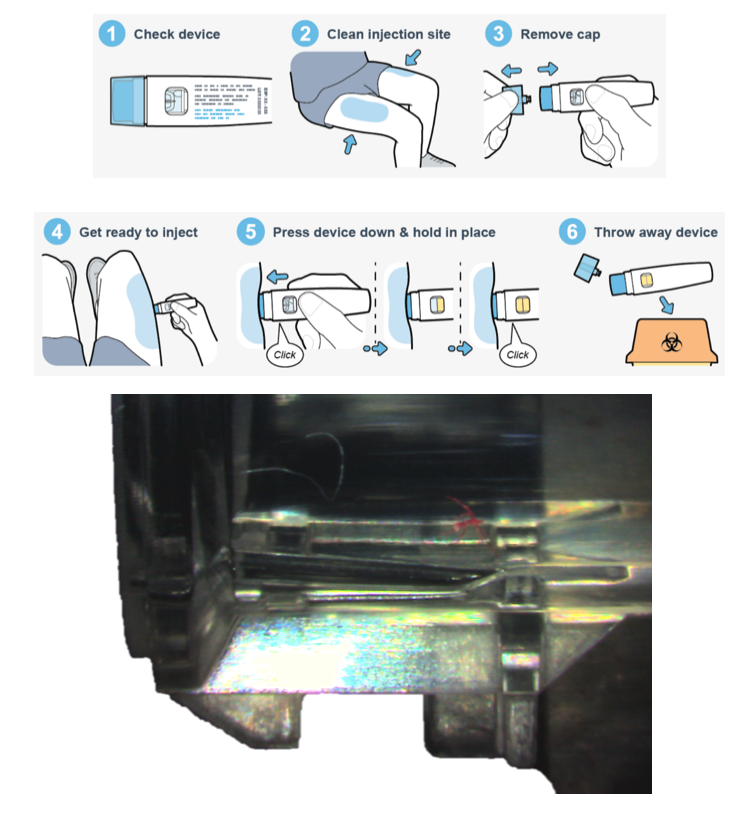
The ease of use of the Sumalen injector is clear from the graphic patient instruction leaflet images seen to the right.
The product was also quiet in use with pleasing haptics – as the clicks are known by geeks like UPC. Small size aided discretion in use, as one cannot control when or where a migraine starts. Rapid injection is needed to stop the symptoms worsening.
Taking a development project into a full-blown medical product is a large leap of management faith, and requires more detail engineering than many people envisage at the start.
A culture change was implemented quietly by UPC to build-up the growing company into a unified team working for the same goals.
One detail requiring attention was different fibres being found in a critical area, that UPC were able to inspect and identify using forensic science.
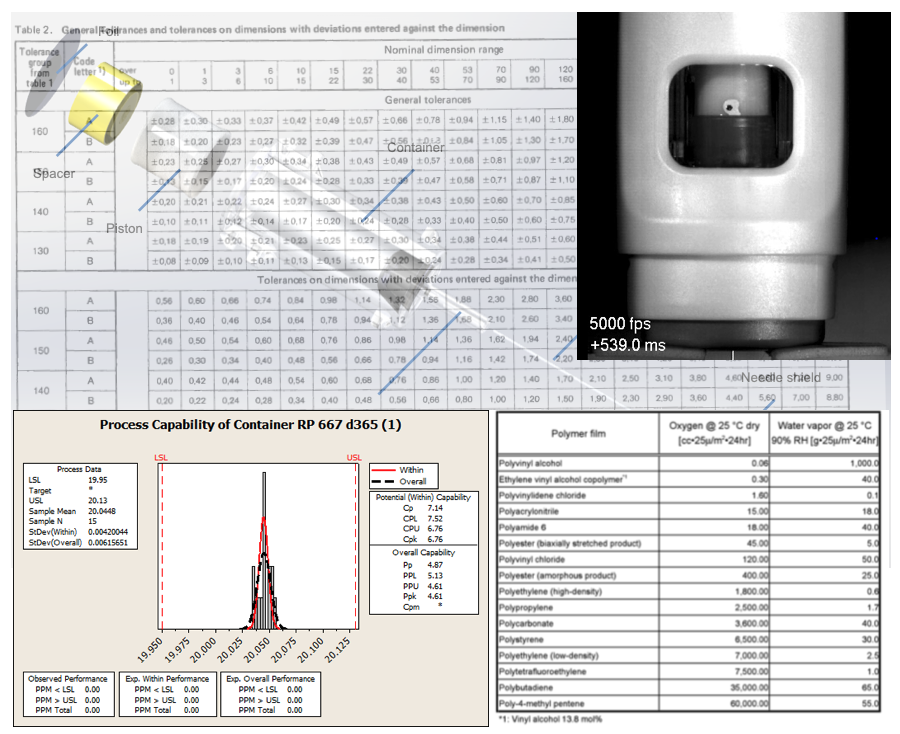
Tolerancing for manufacture; Stability testing; Process Capability
Oval at the time had a six-person team in product R&D working on various projects for the company.
Sumalen was considered a late-stage project nearing design freeze but requiring the following:
- Tolerancing for manufacture in plastic – typical DIN 16901 tables were used by UPC
- Stability testing under accelerated conditions were being undertaken to prove that the mechanisms were robust
- Process Capability of the mouldings was being measured using n=15 on low cavitation tooling – see Minitab output.
Working with the Oval founder and CTO whose creativity knows no end, was interesting every day!
Getting Sumalen market-ready was a great result.

