Technology Insights
Asthma COPD
Small Molecules
Soft Mist Nebuliser
Combination Treatments
Low GWP Propellants
Triples
Biologics
4 Drug Systems?
Gene Therapy
Dry Powder Delivery
B. 1960 Capsule Systems
Montreal Protocol > Powder
Capsule Systems
Factory Metered Systems
Device Metering Systems
Kigali 2019 > Powder
Liquid Active Delivery
b. 1952 PMDI using CFC
Montreal 1985 no more CFC
IPAC Promotes 134a & 227
Breath-Activated pMDI
Counters
Portable Nebulisers
Low GWP 152, 1234
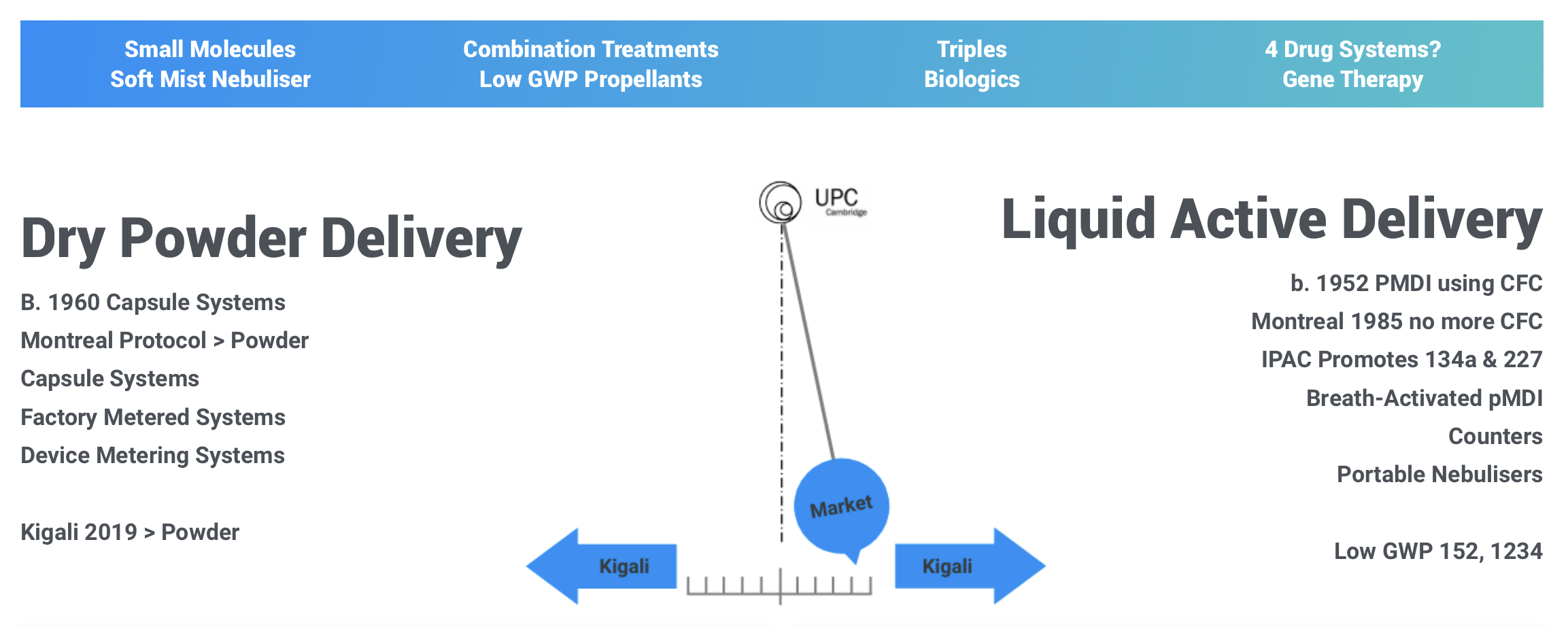
Factory Metered MDPI
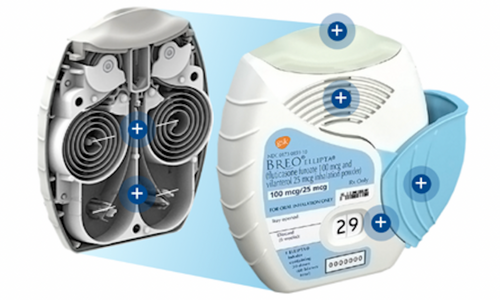
pMDI + Counter pMDI – Press & Breathe
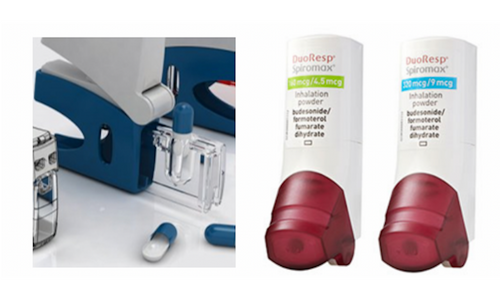
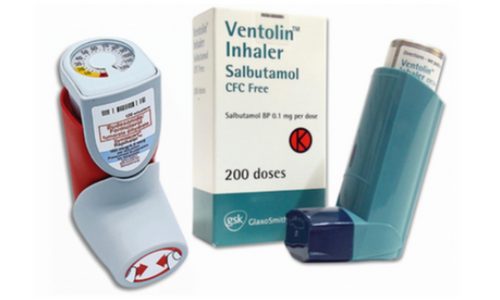
Capsule Device & Device Metering MDPI
Micronised dry powder is most often the active ingredient form for asthma treatments. Capsule systems require dexterity to use; open-inhale-close MDPl’s are more widely acceptable.
Some actives (API) are soluble in HFA so tend to favour the pMDI route, otherwise suspension formulations have to be developed. Liquid metering valve technology using low-GWP propellants is the low-carbon future. DDI breath-activated systems with counters are the most complex delivery DMDI technology electronic mesh nebulisers aside.
Asthma is an allergic reaction by the human body to airborne or contact particles. Daily medicine
treatment is required, generally by deposition in the lung, aside from Merck’s tablet. The lung
route for broncho-dilators using active delivery by propellant or portable nebulisers is effective
upper airway emergency medicine. Inhaler systems are designed to deliver ~100μg in sub 3
micron particles to the lung periphery, there crossing the epithelium into the blood stream.
